Knickerbocker Dream IT
Context
Chemistry A/B is an elective class that is available to students from grade 10 to grade 12. This particular class is geared toward students who are not science-minded and/or do not plan to attend college. While we teach the same standards and benchmarks of a traditional chemistry class, the focus is on everyday experience and practical applications. Also, I have built a reputation in my school for being able to reach and motivate students who have previously been labeled as "intentional non-learners". As a result, my typical class consists of learners who are afraid to take risks, unsure of their abilities, and have negative experiences with teachers and other adults.
The technology infrastructure is in a developmental stage. Some teachers in the science department have printers, document cameras, chemistry probes, and other supplemental devices. However, these are the result of personal classroom grants they have submitted. Most science classrooms, mine included, simply have: an overhead projector (ceiling-mounted), a white-board (non-interactive), a projection screen, and a computer with low processing and speed capabilities.
My classroom is built to accommodate 24 students, based on square-footage and number of lab stations. However, 2 lab stations have standard classroom tables rather than those with fire-resistant tops, presenting one of many safety hazards. Each station is equipped with gas and water access, and shared equipment including standard glassware, bunsen burners, etc.
Technology support is very limited. The district does not seriously consider requests for new computers or additional software or hardware programs, citing budget constraints. Simple requests such as a replacement lightbulb for the projector are delayed for months or longer. Software support is also very slow if/when it occurs.
My classroom is built to accommodate 24 students, based on square-footage and number of lab stations. However, 2 lab stations have standard classroom tables rather than those with fire-resistant tops, presenting one of many safety hazards. Each station is equipped with gas and water access, and shared equipment including standard glassware, bunsen burners, etc.
Technology support is very limited. The district does not seriously consider requests for new computers or additional software or hardware programs, citing budget constraints. Simple requests such as a replacement lightbulb for the projector are delayed for months or longer. Software support is also very slow if/when it occurs.
Content
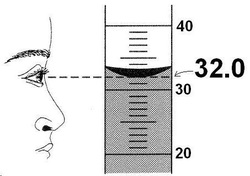
Within the context of significant figures, students are expected to:
Typical problems seen in student mastery of significant figures include:
- Follow a written set of rules to determine how many significant figures are appropriate for each data point.
- Recognize that the number of Sig Figs differs dependent upon the measuring / counting device being utilized.
- Perform calculations based on the quantitative data gathered and express the answers with correct Sig Figs.
- Explain the purpose of significant figures within the context of a high school chemistry class.
Typical problems seen in student mastery of significant figures include:
- Inability to express measurements with correct number of significant figures
- Failure to determine Sig Figs without the list of rules immediately available
- Difficulty checking answers independently (without the use of the textbook or the teacher)
- Frustration with the process and consequent self-defeating attitudes regarding this set of tasks
- Inability to explain errors when an incorrect response is given
- It's important to note that there are very few "misconceptions" present. Most students have never worked with (or even heard of) significant figures before they enter my chemistry class, so incorrect ideas regarding the process are not an issue.
My reasons for choosing significant figures as a particularly difficult topic in chemistry is based on the following:
- Student assignment and assessment scores show a marked decrease during the Sig Fig unit.
- Parents frequently express concern about the content and their student's difficulty in meeting the expectations.
- Other science teachers in the district have chosen not to teach significant figures in recent years, since it is no longer a state requirement. These same teachers concede that this is a basic and important part of high school chemistry, but cite student learning difficulties as their rationale for dropping the unit.
- Teachers at annual Michigan Science Teachers Association (MSTA) and National Science Teachers Association (NSTA) meetings report the same student difficulties with significant figures, but have little to no offerings as to how to resolve them.
Technology
The technology best-suited to teaching and learning significant figures is the graphing calculator, equipped with a Sig Fig program. Below are two other options I researched and considered:
1. Online games and practice sets
Issues presented by this option: Answers can be counted as incorrect based on the formatting of the number inputted by the student. Also, it's easy to make mistakes on Sig Figs because of the complexity of the rules. Therefore, many online resources, even those published by high school and college-level instructors, contain incorrect feedback that can be extremely confusing and frustrating for students. Lastly, not all students have access to computers. Laptops can be checked out and used in class, but are not available on a daily basis.
2. Response systems
2. Response systems
Issues presented by this option: Setting up the question-and-answer sets for a response system is time-intensive. Also, the software required by this type of system slows down computers that are already "chugging along" due to system age as well as outdated operating systems. Additionally, East Lansing Public Schools do not provide technical support for any software or hardware that they did not install. I considered this particular option heavily, and would definitely consider using it in conjunction with the graphing calculators in the future.
Graphing calculators are the best option for the classroom, as the margin of error is exceptionally low when compared with the other options above. Also, every student would have equal access to the resource, unlike online learning games. There is also little set-up, and that which does exist is only needed once and is then ready-to-go from that point forward. Further, students who wish to do so can purchase their own graphing calculator for use at home. (These are available used for $10-15). Also, students are familiar and comfortable with the idea of a calculator. The technology itself would not be intimidating or slow progress down with a learning curve of the resource itself. As noted above, however, I would strongly consider integrated a classroom response system with the class set of graphing calculators in the future.
Graphing calculators are the best option for the classroom, as the margin of error is exceptionally low when compared with the other options above. Also, every student would have equal access to the resource, unlike online learning games. There is also little set-up, and that which does exist is only needed once and is then ready-to-go from that point forward. Further, students who wish to do so can purchase their own graphing calculator for use at home. (These are available used for $10-15). Also, students are familiar and comfortable with the idea of a calculator. The technology itself would not be intimidating or slow progress down with a learning curve of the resource itself. As noted above, however, I would strongly consider integrated a classroom response system with the class set of graphing calculators in the future.
Pedagogy
The Sig Fig graphing program functionality is a beautiful bridge between our most important teaching goals and integration of technology. I struggled with the choice of a tool that is so basic and familiar to address a common and serious teaching challenge in chemistry. However, the simplicity of the solution does not detract from its value. Many teaching and learning theories apply here, but it seems that the most clear and basic example best illustrates the application of this familiar and basic tool:
Maslow's Hierarchy of Needs:
We much first meet a persons more basic needs
Before they are emotionally and psychologically able to access and achieve more complex needs:
This technological learning tool offers a feeling of insulation from the risk of offering a numerically "wrong" answer. It instead shifts the efforts toward building your base knowledge in order to use the tool, and explaining WHY a calculation results in a particular number of Sig Figs. This all lends to an increased sense of belonging and community as well as increased peer consulting. In turn, students are able to achieve their fullest potential in the class.
We much first meet a persons more basic needs
- Sense of belonging
- Safety and security
- Physiological
Before they are emotionally and psychologically able to access and achieve more complex needs:
- Self-esteem and recognition
- Self-actualization and realization of potential.
This technological learning tool offers a feeling of insulation from the risk of offering a numerically "wrong" answer. It instead shifts the efforts toward building your base knowledge in order to use the tool, and explaining WHY a calculation results in a particular number of Sig Figs. This all lends to an increased sense of belonging and community as well as increased peer consulting. In turn, students are able to achieve their fullest potential in the class.
The Total PACKage
As science educators, we can easily become entrenched in "right answers" and "sound explanations" for the topics we cover. It's shamefully simple to forgo the exploration of inquiry for the more straightforward and direct delivery of information in the form of lectures and demonstrations. We fail to consider the primary impulses for learning that are integral to a rich and lasting experience.
- Communication: Significant figures is first and foremost a means of communicating the precision and accuracy of a quantitative measurement to other scientists. Also, student-to-student interactions are fostered through the ability to self-check and therefore lend assistance to another student. Students no longer need to seek assistance from their textbook, an online resource, or even the teacher! Self-reliance and the ability to teach one another develop directly through the availability and use of this tool.
- Inquiry: Because the Sig Fig graphing program only provides answer for calculations and not individual data points, this tool does not provide "all the answers" in the context of significant figures. In order for any calculation to produce a correct answer, the data inputted must contain appropriate significant figures. This tool does not remove the need for careful measurement and the ability to apply Sig Fig rules.
- Expression: The willingness to share answers, participate in class discussion, and seek out peer-to-peer interactions is made much easier when a student's confidence is high. Having a resource to check answers at the end of a calculation lends to that goal directly. The mood of a classroom is much less stressful and boring when the group functions as a learning family, rather than a lecture hall.
- Construction: The graphing calculator Sig Fig program allows the student to perform calculations with that descriptive data and produce answers that are equally (not more, not less) accurate than the original numbers.
Transformational Technology Applications
- The sense of community that can be built by this simple tool is something that is desperately needed in chemistry classes. They are too dependent upon the teacher as the primary source of information dissemination. Taking the onus off of the teacher and placing it squarely upon the shoulders of the learners allows for personal growth and a true learning community. My students often catch mistakes in textbooks, online applets, and sometimes even my own statements. This will add to that sense of equality among learners. Once students start asking for and offering help amongst themselves, peer review and collaboration are the natural sequence. We need tools like this in the classroom, to raise our expectations of students and in turn raise the satisfaction of them as learners.
- Raising the bar of expectation does more for our students than increase their social and academic networking skills. As we covered in Maslow's Hierarchy of Needs, it also allows for self-actualization. What this means is that students are able to achieve in the most profound ways possible because their basic needs are met. Before the application of the Sig Fig graphing program, many students were unable to move beyond applying Sig Fig rules to individual quantitative measurements. Now they can use this tool to input those measurements into calculations and explore the effects created when 2 seemingly opposing Sig Fig numbers interact. They can move beyond memorization and rule-following into application and inquiry. Students could be given the calculator and NO rules and be assigned the task of figuring the rules out themselves! The significance of this is astounding. No chemistry class should be without tools, like this one, that enable teaching and learning to reach new levels of achievement.